Regularly in Standard Operating Procedures, FDA’s Title 21 CFR Part 11 regulations, EMA’s Eudralex Volume 4 Annex 11 guidelines for industry, and ISPE’s Industry Best Practice GAMP 5 are mentioned in a single sentence. More often then not, people are not fully aware of what the differences are between Part 11, Annex 11, and GAMP 5.
With this post, I am sharing a tabular overview of all three and makes a detailed comparison between the three documents, where they align and where the differ.
Since late 2022 GAMP 5 Version 2 has been published and this tabular overview is not fully up to date any longer. If you are interested in the updated version, let me know and I can email you the excel table.

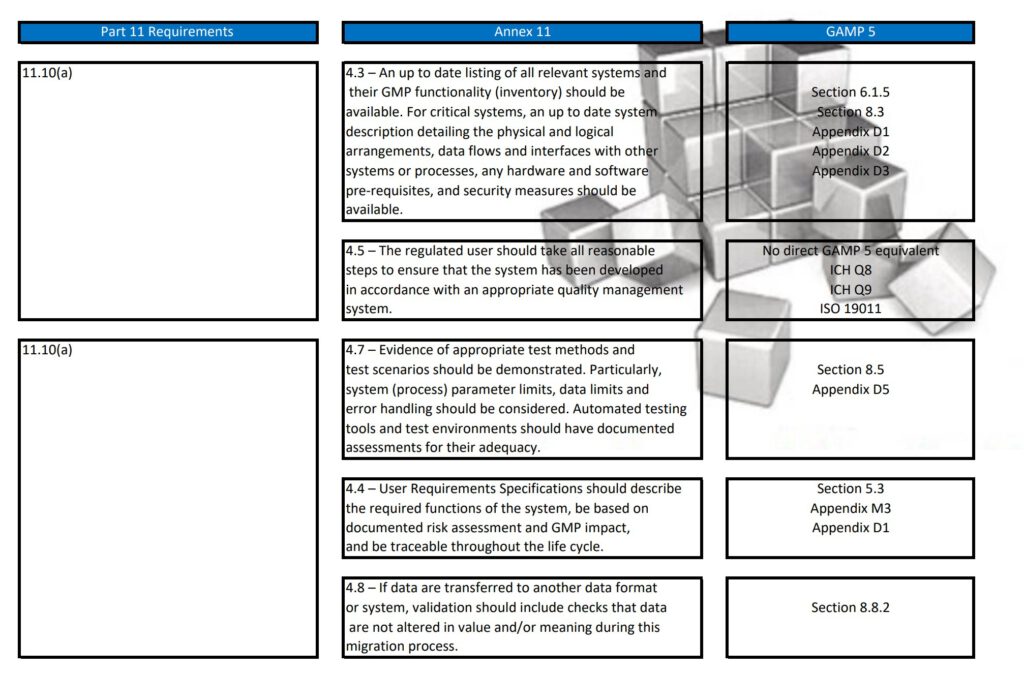

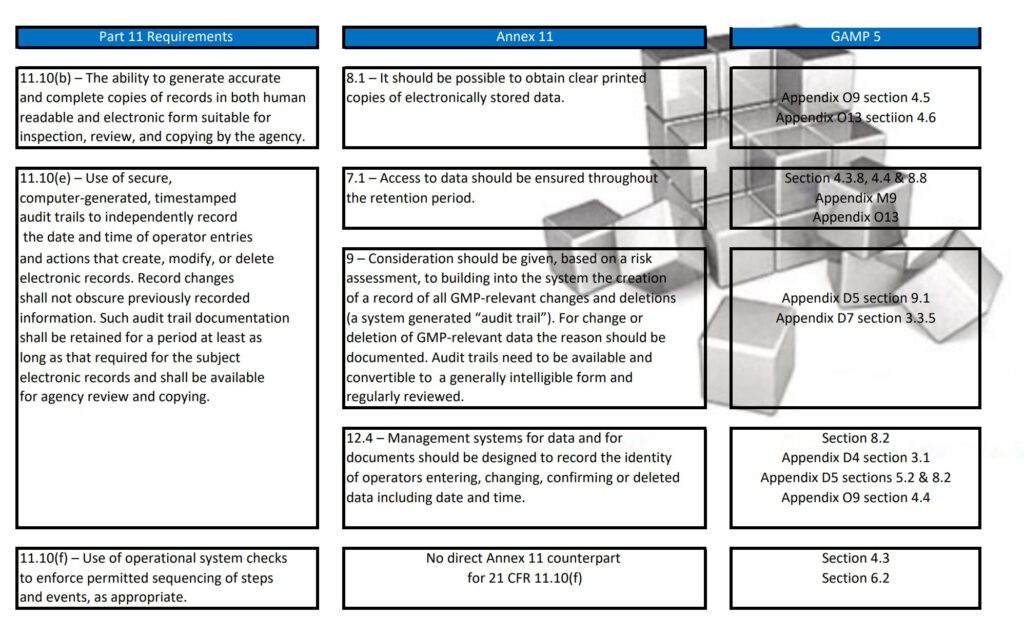
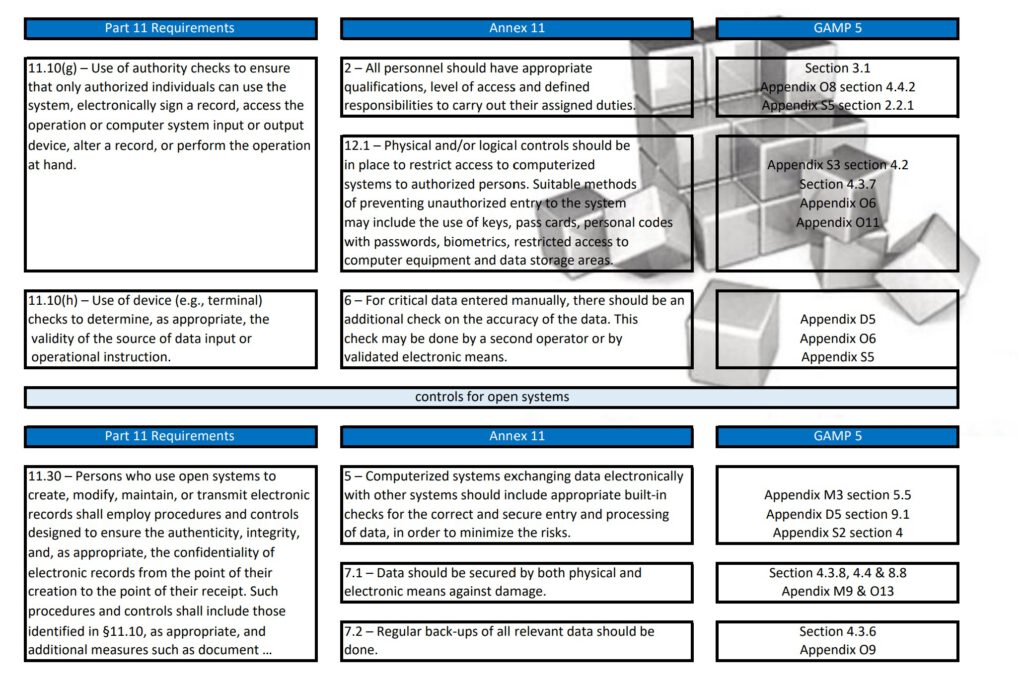
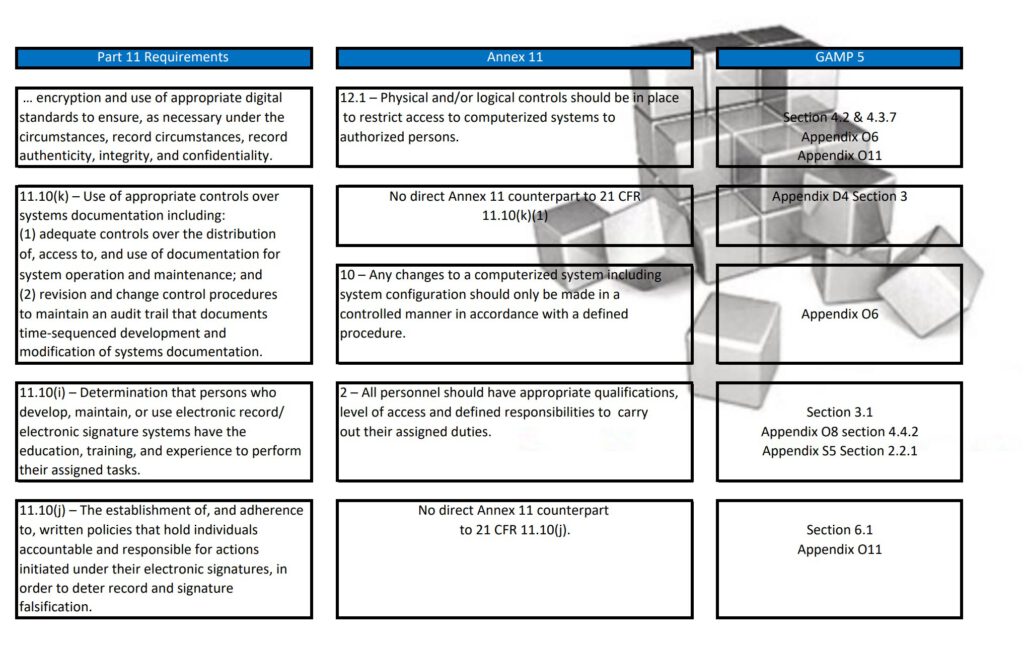


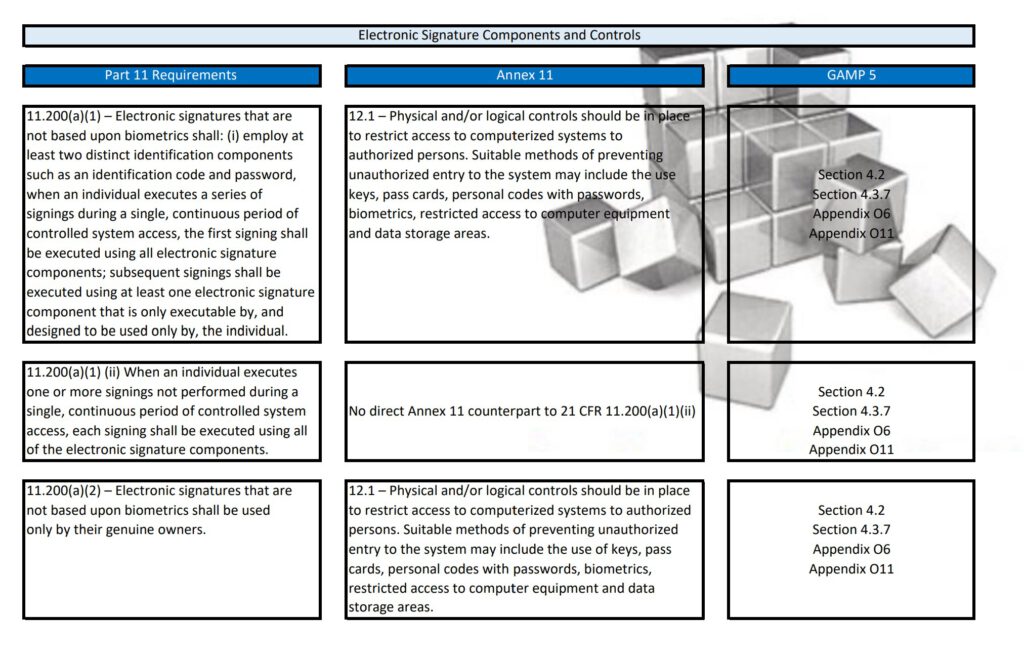
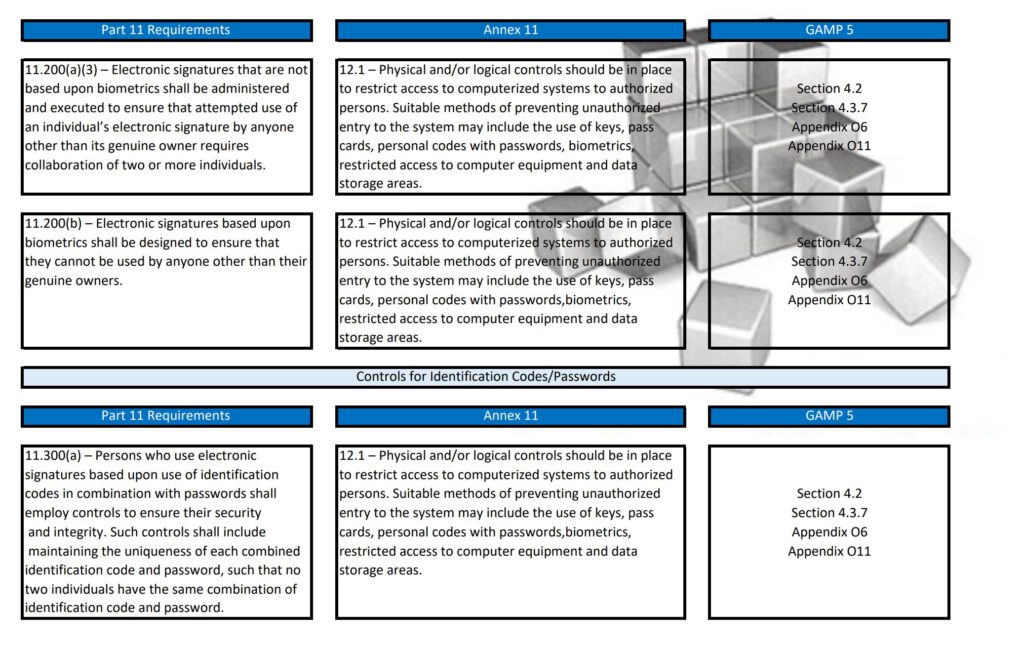
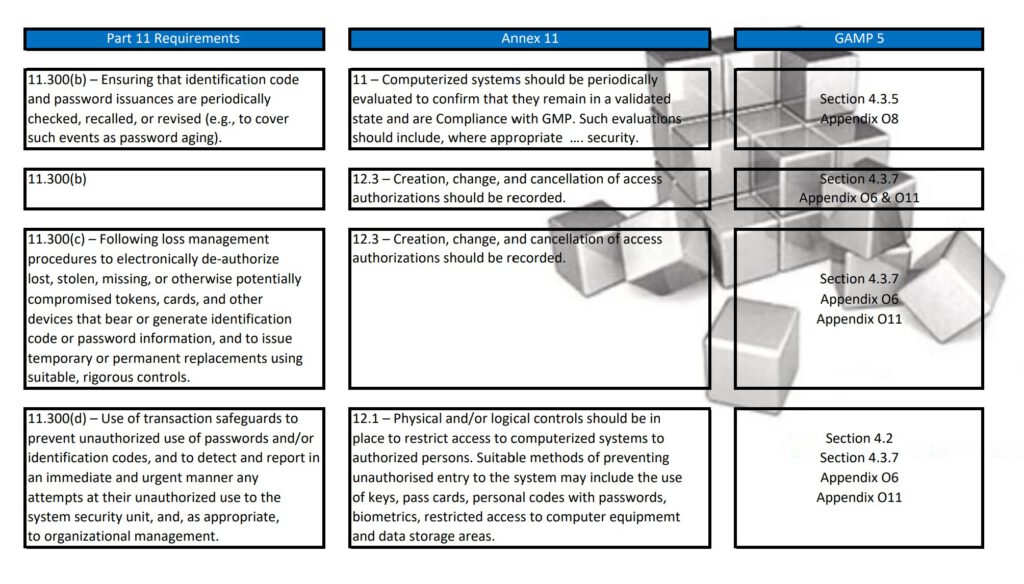
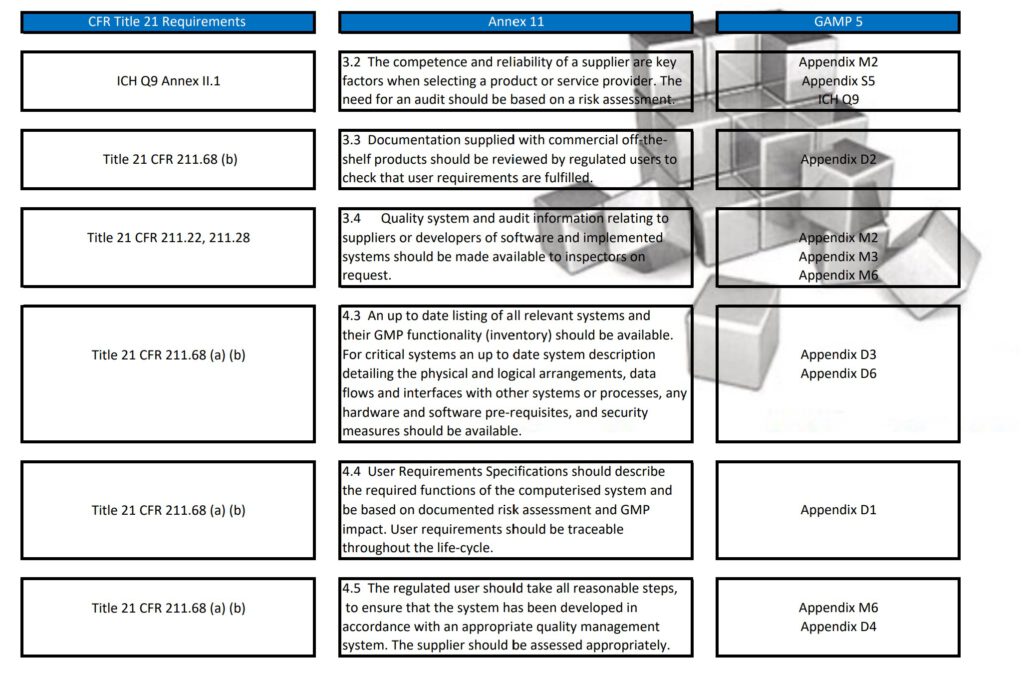
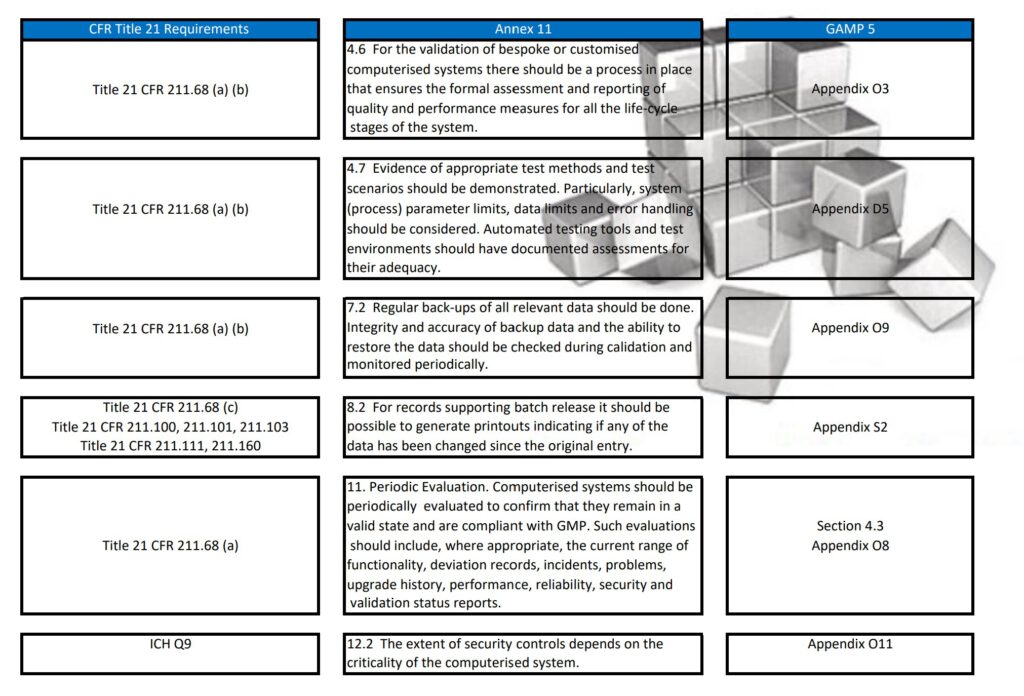
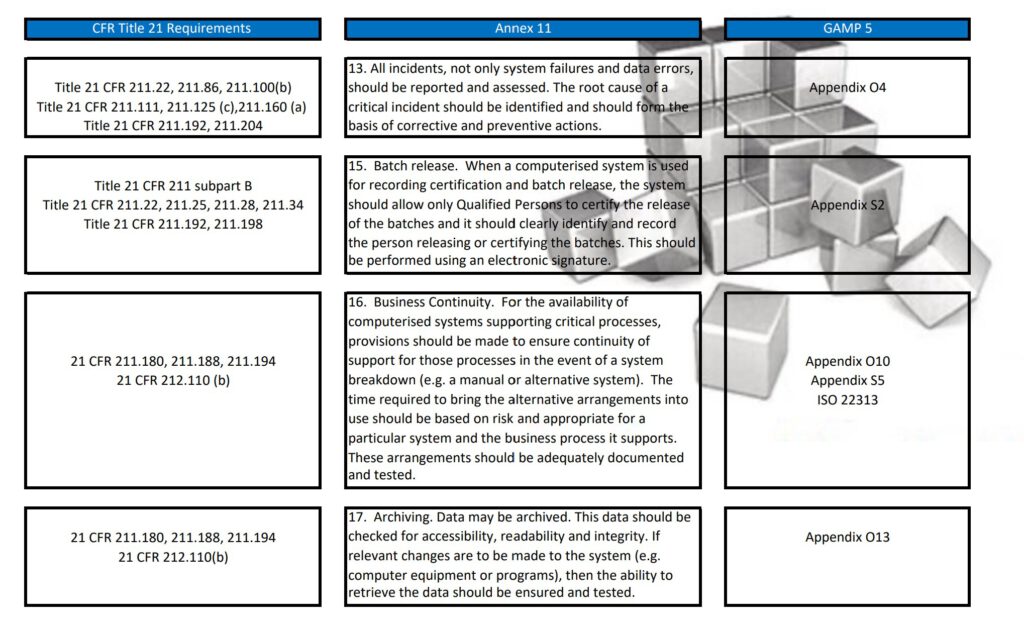
Besides Part 11, Annex 11, and GAMP 5, also ICH Q9, and some ISO guidelines have been referenced and all are relevant to consider when scoping, designing, implementing, and operating computerized systems.
