cGMP can mean different things to different people. In this short article, I touch upon some historical occurrences that helped shape cGMP as we know to date. A full one-day or multi-days training, geared towards the audience does go into depth and with more detail then I want to do here.

When working in cGMP, the GMP Golden Rules or the 10 Commandments, may have been mentioned or taught; should not be unknown. New to cGMP, you may want to take a moment to read and understand what is stated with the Golden Rules or the 10 Commandments. Summarized in just two sentences, cGMP can be captured as “What I do is defined entirely by how well I follow my procedures and document the outcomes. Science is the bedrock upon which I rely for all decisions”. More briefly also: “Say what you do, do what you say, demonstrate it!”
Further in this article, I will present some striking examples that have led to more clear, more strict, and more detailed legislation and guidelines for drugs.

Serum obtained from a with diphtheria infected horse, which also, unknowingly was infected with tetanus, was used as an antitoxin to treat patients, which then unexpectedly died of tetanus. When this was discovered not just in St. Louis but also elsewhere, it did lead to the Biologics Control Act in 1902.

Back in the early 20th century, the legislation for Food and Pharma was even less differentiated compared to today and Congress, in 1906, was motivated by negative press coverage about (poisonous) additives in Food, to pass the Pure Food & Drug Act.
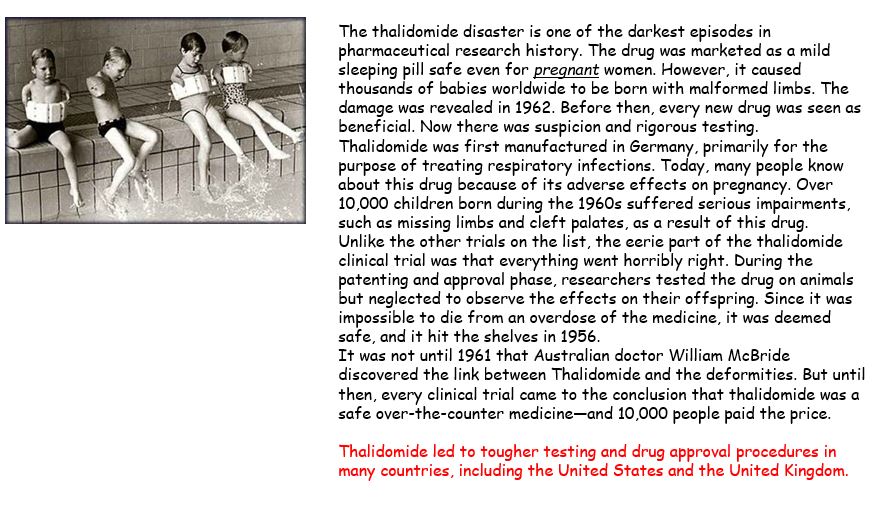
Pharmacologist Frances Oldham Kelsey turned down several requests from the distributing company who did not provided clinical evidence to refute reports of patients who developed nerve damage in their limbs after long-term thalidomide use. This prevented the drug thalidomide from ever being used in the United States. Thalidomide had a devastating effect outside the USA but across the world led to much tougher testing and drug approval procedures. One key change was that drugs intended for human use could no longer be approved purely on the basis of animal testing. And drug trials for substances marketed to pregnant women also had to provide evidence that they were safe for use in pregnancy.
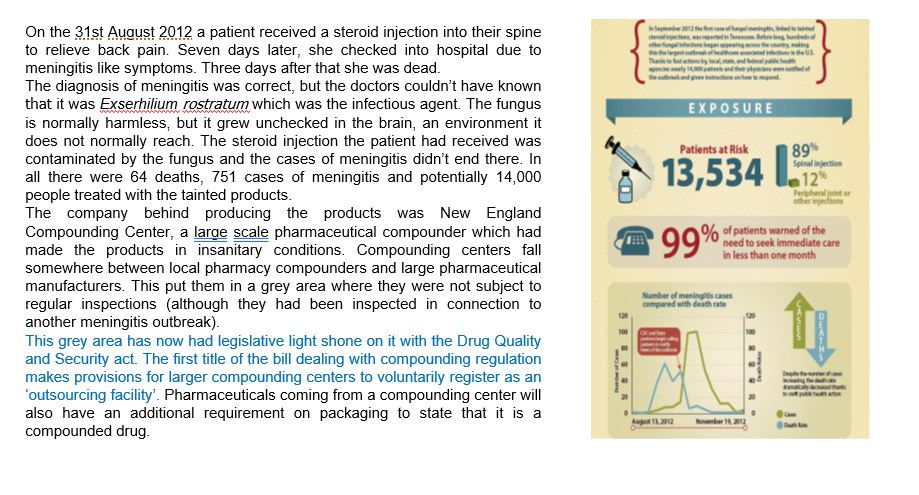
This example of compounding dramatically shows that not only the purity of the raw materials and excipients is of importance but equally the cleanliness of equipment and facilities.

Packaging & labelling and line-clearance are important aspects, too. They need tight controls to ascertain the correct packaging, labelling but also to make sure excess packaging & labelling materials are appropriately accounted for, discarded or reconciliated to avoid mistakes or misuse.

From the first moment in 1202 where King John proclaimed the adulteration of bread with ingredients such as ground peas or beans unlawful (the Assize of bread) until today, this timeline provides a high-level overview of the guidelines and regulations since 1820 in the USA. Still today, new regulations come into effect.
This article touches only on some of the striking examples why regulations serve an important purpose and more importantly what we can absorb from those experiences to intrinsically know what we need to do to manufacture the right drugs correctly. A single or multi-day training on this topic has a broader reach and depth and will touch upon all the different departments in a manufacturing environment, from sourcing, supply, receipt, quarantine, delivery, production, inspection, testing, release, storage, to finally transportation to the hospital or pharmacist to deliver to the patient.
