Also for Maintenance in Biotech/Pharma
Maintenance in Biotech/Pharma environment is always embedded in the Quality Management System. As such, maintenance is also subject to regulatory requirements.
This short article shows how Maintenance can be implemented in the regulatory environment common to Biotech/Pharma and at the same time be comprehensive and lean.
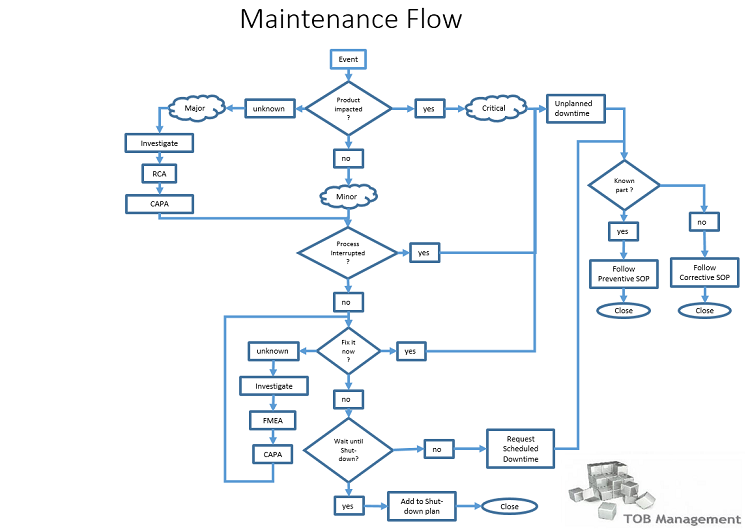
In this simplified representation, maintenance can be described in four (4) basic procedures; the umbrella procedure for Maintenance, an SOP for Preventive Maintenance, another SOP for Corrective Maintenance, and lastly a procedure for (recurring) Shut-downs.

The umbrella SOP (1) describes the flow diagram above and gives appropriate reference(s) to required investigations for product impact and also needed investigation into the technical necessity to fix the event.
The Preventive Maintenance SOP (2) describes what Preventive Maintenance is, how Preventive Maintenance is decided, initially and ongoing, but also how and who implement Preventive Maintenance and finally, report on the outcome of the activity.
The Corrective Maintenance SOP (3) (describes what Corrective Maintenance is, how Corrective Maintenance is decided and when a switch to the Preventive Maintenance is justified (known part with unexpected failure) when to continue to follow the Corrective Maintenance Sequence (unknown part unexpectedly failed) and what is expected to be done before actual Corrective Maintenance can commence (QA approval).
The Shut-down SOP (4) describes how a Shut-down is prepared, what the conditions are for an activity to qualify for the Shut-down, what is required for main & sub-tasks on the Shut-down list, what is needed; what needs to be arranged, who needs to agree, approve, authorize and what & how reporting is done at the close-out of a Shut-down.
Next to the SOPs (1-4), some Work Instructions and Forms need to be developed. Work Instructions are required for all those maintenance tasks and maintenance parts that have been identified to require periodic service, either for cleaning, inspection, lubrication, or replacement. Forms are needed to document those activities.
Within the SOPs, references will be made to other SOPs within the Quality Management, such as Change Control, Deviation Management, but also Risk Management, Impact Assessment, and tools like Root Cause Analysis and Failure Mode and Effect (& Criticality) Analysis.
A comprehensive and lean Maintenance Management System as proposed in the article is easy to maintain, does not require much time for (recurring) training, can be deployed quickly, and is easy to stay compliant.
